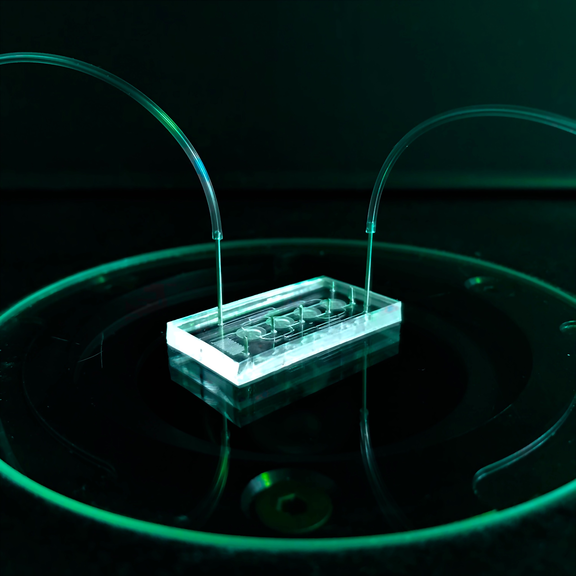
Organ-on-Chip

Next Generation Cellular and Organotypic Assays
Senior Principal Scientist
In the Organ-on-Chip group, we are engaged in the development and qualification of Organ-on-Chip (OoC) systems and enabler technologies, as well as the application of OoC models for basic research, drug development and pharmacological research, personalized medicine, consumer protection and toxicology. With our international team, we work at the interdisciplinary interface between materials science, engineering, physics, biology and medicine.
Organ-on-chip systems are small microfluidic platforms that integrate living substructures of organs into a controlled microenvironment and replicate one or more aspects of the organ's in vivo dynamics, functionality, and (patho)physiology. In this way, complex human biological processes can be physiologically simulated outside the human body. The small three-dimensional chambers and micrometer-scale channels integrated into the chips, as well as special geometric, mechanical, and biological properties and components, can mimic the natural physiologically perfused microenvironment of cells in a tissue. Organ-on-chip systems consequently combine the unique selling points of classical cell assays (human cells and genes) and animal models (complex 3D tissues and blood circulation). These so-called in vitro test systems can be used to answer a wide variety of medical, biological, pharmacological and toxicological questions without having to resort to laboratory animals.
In close cooperation with the 3R-Center Tübingenfor in vitro models and animal testing alternatives, we thus offer the possibility to reduce the use as well as the necessity of animal testing according to the guidelines of the 3R principle (Replace, Reduce, Refine), to increase the transferability of preclinical results to the clinical phases and thus to make the entire development more cost-effective, safer and faster.
Development of testing systems
- Design and development of microfluidic solutions for cell applications
- Combination of microfluidics, biomaterials and 3D tissues
- Development of microphysiological organ-on-a-chip systems
- Functional testing and validation of in vitro models
- Development of fluidic solutions for cultivation and differentiation of stem cells
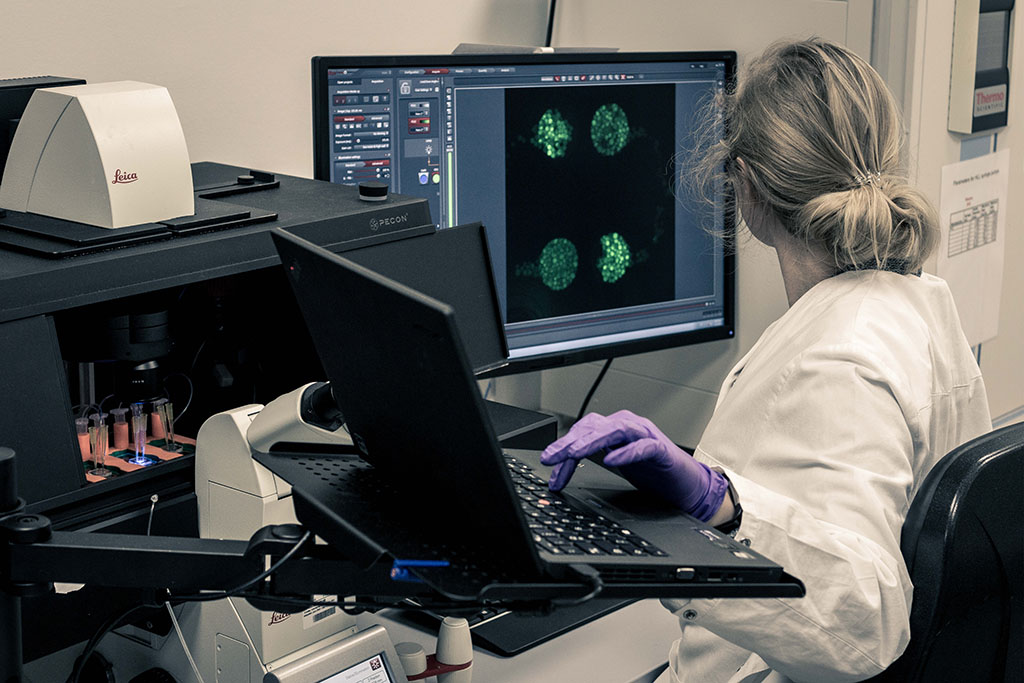
Evaluation of external microfluidic platforms
- Establishment of microphysiological tissue models and assays in external microfluidic platforms
- Validation of phyiological cultivation parameters
- Determination of viability and functionality of tissue units
- Establishment of endpoints and assay developments
- Substance testing
Techniques, methods, equipment
- Chip fabrication labs with a wide variety of patterning and joining methods such as lithography, laser cutting, hot stamping, 3D printing, thermo-mechanical joining and laser welding
- Microfluidic application lab for chip culture and simultaneous documentation
- Cell culture laboratories for stem cell work and genetic engineering work
- Isolation of a range of human cell types and differentiation of stem cells
- Functional assays: including cytotoxicity, proliferation, apoptosis
- Read-outs of media supernatants from capillary channels as well as the direct cellular environment
- Provision of samples extracted from chips
for protein expression - RNA expression
- Immunohistochemistry of organs-on-chips
- Gene expression analysis
- Wide-field and confocal microscopy, fluorescence microscopy
- Live cell imaging and image analysis
- Fluorescence microscopy for quantitative and qualitative analysis of cell interactions in chips
- Fluorescently labeled proteins (e.g. for analysis of living cells)
- GFP expression
- Quantitative and qualitative fluorescence microscopic analysis of formed 3D microtissue structures





