
Organoid electrophysiology and mesh microelectrode arrays

Next Generation Cellular and Organotypic Assays
Senior Scientist
Advanced solutions for organoid, spheroid and tissue models
Our mesh microelectrode arrays (mesh MEAs) are optimal tools for researchers studying electrophysiology of 3D neural and cardiac tissue models. Designed for non-invasive, long-term electrophysiology, our mesh MEAs integrate within spheroids and organoids, for straightforward recording and stimulation inside the tissue.
Benefits of mesh MEAs
- 3D Compatibility: The mesh structure gently suspends organoids away from any rigid surfaces. The hammock-like mesh initially supports the organoid, and over time the cells grow through and around the mesh, resulting in electrodes embedded within the tissue. In contrast, traditional planar MEAs contact only superficial cells, restrict nutrient and gas exchange, and disturb the 3D organoid shape.
- Long-Term Viability: Designed for months-long culture, our devices enable longitudinal studies of electrical activity or chronic exposure or disease models. Organoids can be transferred to the mesh MEA at any stage of development.
- Scalable & Easy to Use: We produce mesh MEAs using state-of-the-art microfabrication methods designed for efficient production with high yield. Our MEAs are designed for use with commercial amplifiers and fluidic handling systems.
- High-Resolution Data Acquisition: Our low-impedance microelectrodes are optimized for low-noise recordings, capturing spontaneous and evoked activity with precision.
Applications
- Functional characterization of brain organoids and neural spheroids
- Electrophysiological studies in cardiac spheroids and engineered heart tissues
- Pharmacological screening and toxicology testing in 3D cellular models
- Development of personalized medicine and disease modeling platforms
Porous, thin-film polyimide MEAs were developed at NMI in the early 2000s for improved interfacing with in vitro tissue preparations (Stett et al., 2007) and are available as pMEAs from Multi Channel Systems. Our polyimide technology was developed in part to support clinical retinal implants (together with the now-defunct Retina Implant AG), and have been broadly used for implantable devices (Jones et al., 2016; Pascual et al., 2023; Steins, 2023), including porous options to improve interfacing with tissue.
Elsewhere, mesh MEAs for in vitro experiments were first reported in 2012 (Tian et al., 2012). This work was an early step towards a large body of work on implantable mesh MEAs (described as “mesh electronics” by the lab of Charles Lieber and others) (Hong et al., 2018). Mesh MEAs use polymer structures with cellular-scale feature sizes, thereby minimizing mechanical mismatch to cells and tissue, minimizing foreign body response, and optimizing recording quality and longevity.
With the rise of neural organoids in the early 2010s, the application of mesh MEA concepts to these 3D in vitro models was logical. A primary challenge for mesh MEAs, whether in vitro or in vivo, is the electrical packaging or connectors to external electronics. For in vitro mesh MEAs, another challenge is well designs for simple handling and medium exchange, as well as compatibility with sterilization and long-term stability. An organoid-specific MEA was a common request, whether from attendees at the MEA Meeting or from our colleagues in the Electrophysiology and Molecular Neurobiology groups at NMI.
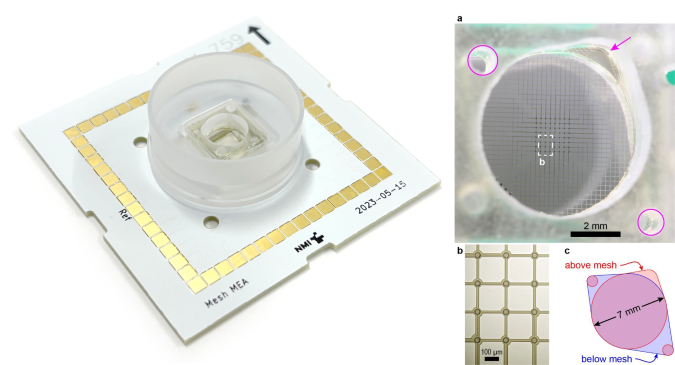
In close collaboration with the Max Planck Institute for Molecular Biomedicine under the supervision of Dr. Thomas Rauen and Prof. Hans Schöler, we developed a cutting-edge mesh MEA platform. This innovative approach was specifically designed to advance proof-of-concept experiments, seamlessly integrating mesh MEAs with neural organoids to push the boundaries of neurobiological research (McDonald et al., 2023). More recently, we have developed mesh MEAs with advanced packaging methods to improve manufacturing scalability, including versions using wire-bonding of polyimide chips (Stumpp et al., 2023) and improved well designs (above images).
We continue to be inspired by and learn from excellent work by the international MEA community. Other solutions designed for spheroids have been reported (Kireev et al., 2019; Li et al., 2019; Kalmykov et al., 2021; Le Floch et al., 2022; Yang et al., 2024). This list is not meant to be exhaustive, and we’re always interested in learning about the latest technology!
Hong, G. et al. (2018) ‘Mesh electronics: a new paradigm for tissue-like brain probes’, Current Opinion in Neurobiology, 50, pp. 33–41. Available at: doi.org/10.1016/j.conb.2017.11.007.
Jones, P.D. et al. (2016) ‘In vitro and in vivo probes with mushroom-shaped microelectrodes - tools for in-cell electrophysiology’, in 10th International Meeting on Substrate-Integrated Microelectrode Arrays. Reutlingen. Available at: doi.org/10.3389/conf.fnins.2016.93.00076.
Kalmykov, A. et al. (2021) ‘Bioelectrical interfaces with cortical spheroids in three-dimensions’, Journal of Neural Engineering, 18(5), p. 055005. Available at: doi.org/10.1088/1741-2552/abf290.
Kireev, D. et al. (2019) ‘N3-MEA Probes: Scooping Neuronal Networks’, Frontiers in Neuroscience, 13, p. 320. Available at: doi.org/10.3389/fnins.2019.00320.
Le Floch, P. et al. (2022) ‘Stretchable Mesh Nanoelectronics for Three‐Dimensional Single‐Cell Chronic Electrophysiology from Developing Brain Organoids’, Advanced Materials, p. 2106829. Available at: doi.org/10.1002/adma.202106829.
Li, Q. et al. (2019) ‘Cyborg Organoids: Implantation of Nanoelectronics via Organogenesis for Tissue-Wide Electrophysiology’, Nano Letters, 19(8), pp. 5781–5789. Available at: doi.org/10.1021/acs.nanolett.9b02512.
McDonald, M. et al. (2023) ‘A mesh microelectrode array for non-invasive electrophysiology within neural organoids’, Biosensors and Bioelectronics, 228, p. 115223. Available at: doi.org/10.1016/j.bios.2023.115223.
Pascual, D. et al. (2023) ‘A flexible implant for acute intrapancreatic electrophysiology’, Biomedical Microdevices, 25(3), p. 35. Available at: doi.org/10.1007/s10544-023-00662-2.
Steins, H. (2023) A flexible protruding microelectrode array for visceral neuromodulation. 1. Auflage. München: Verlag Dr. Hut (Biomedical microtechnologies, volume 27).
Stett, A. et al. (2007) ‘Improving of signal-to-noise ratio of electrophysiological recordings from explanted retinas by using perforated microelectrode arrays’, in Mikrosystemtechnik Kongress. Mikrosystemtechnik Kongress, Dresden: VDE Verlag GmbH.
Stumpp, T. et al. (2023) ‘Scalable mesh microelectrode arrays for neural spheroids and organoids’, Current Directions in Biomedical Engineering, 9(1), pp. 575–578. Available at: doi.org/10.1515/cdbme-2023-1144.
Tian, B. et al. (2012) ‘Macroporous nanowire nanoelectronic scaffolds for synthetic tissues’, Nature Materials, 11(11), pp. 986–994. Available at: doi.org/10.1038/nmat3404.
Yang, X. et al. (2024) ‘Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids’, Nature Biotechnology [Preprint]. Available at: doi.org/10.1038/s41587-023-02081-3.





