Testing of Viral Systems for Gene Therapy

Next Generation Cellular and Organotypic Assays
Senior Principal Scientist
Characterization of viral systems for gene therapy - efficacy testing using AAV as an example
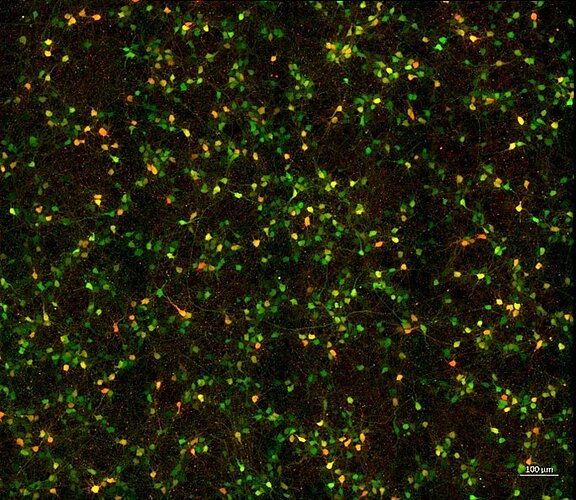
Adeno-associated viruses (AAV) represent suitable tools for the development of safe gene therapy approaches due to their defined tropism and their property of not causing permanent changes at the genome level after infection of the target cell.
The NMI supports its clients both in an initial demonstration of the functionality of newly developed therapeutic AAV and in the development of suitable, throughput-capable cellular test systems to determine the efficacy of different production batches.

Our services
- Use of cellular test systems for validation of tropisms and quantitative determination of infectious titers
- Verification of the functionality of applied AAV by means of gene expression analyses and/or functional assays
- Development of "potency assays" for routine determination of the efficacy of different production batches
Methods
-
Confocal fluorescence microscopy
-
Flow cytometry
-
Quantitative gene expression analysis (qPCR, Western blot)
-
ELISA, homogeneous time resolved fluorescence (HTRF)
Instruments
-
Flow cytometry/FACS: BD FACSmelody, BD LSR Fortessa
-
Microscopy: Carl Zeiss CLSM + Cell Observer SD, Molecular Devices ImageXpress Micro confocal
-
Western Blot: Typhoon Trio imager, LI-COR Odyssey
-
Quantitative real-time PCR: Applied Biosystems StepOnePlus
-
ELISA, HTRF: Tecan Spark, BMG Labtech PHERstar





