Biomarker

BioDevCenter
Principal Senior Scientist
Indicators for diagnosis and therapy
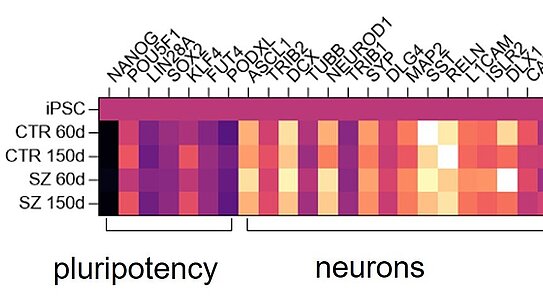
Quantitative detection of a variety of biomarkers with simultaneous high sensitivity and specificity is a challenge that can be solved today using modern technologies in combination with immunoassays. For example, single target analytes can be quantitated at extremely low concentrations using ultrasensitive sandwich immunoassays. Other immunoassay formats allow detection of hundreds of target analytes from minute sample volumes. In any case, it is important to validate the assays before they are used in screening programs.
Development and validation of immunoassays
At the NMI, more than 35 interdisciplinary scientists, engineers and technical staff work on the development and validation of immunoassays on different platforms, such as the bead-based Luminex technology, the ultrasensitive SIMOA sandwich immunoassay platform from Quanterix, our proprietary multiplex DigiWest technology or reverse phase protein arrays.
Important topics in publicly funded projects or direct contracts are drug development for effect and side effect analysis or biomarker qualification in drug-induced organ damage.
Learn more details about the subtopics here: